Cluster Headache Dosing & Administration
Recommended dosing with no titration required1a
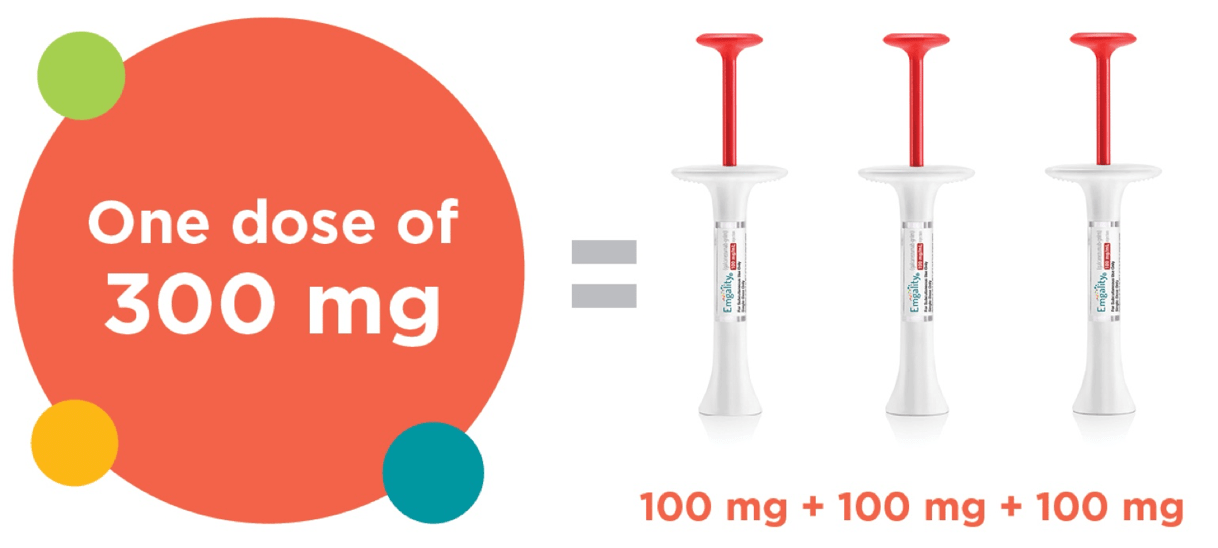
300 mg administered as 3 consecutive SC injections of 100 mg each, at the onset of the cluster period, and then monthly until the end of the cluster period.
- 300 mg dose; this comes in 3 x 100 mg prefilled syringes
- These 3 syringes are administered subcutaneously, one after another
- Patients begin treatment at the onset of a cluster period
- Patients take this 300 mg dose every month until their cluster period ends
Please review full Instructions for Use with your patients.
aThe Emgality prefilled syringe needle is 27 gauge x ½ inch.2
Consider having patients fill their prescription for their upcoming cluster cycle while they are in remission.
Advise patients to1,2:
- Store product refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect it from light until use
- Check expiration date on product prior to use
SELECT IMPORTANT SAFETY INFORMATION
Contraindications
Emgality is contraindicated in patients with serious hypersensitivity to galcanezumab-gnlm or to any of the excipients.
How to administer Emgality
Preparation3
- Inspect the prefilled syringe and the medicine. Do not use the prefilled syringe and dispose of it if it looks damaged; the expiration date printed on the label has passed; or the medicine is cloudy or discolored, has small particles, or is frozen
- Choose an area for injection: abdomen or thigh if self-injecting, and buttocks or back of upper arm if another person is injecting. Be sure to choose a different site (even within an area) for each injection
- Clean the site with an alcohol wipe and let it dry before injecting
3 key administration steps3
Please review the full Instructions for Use with your patients to ensure they understand how to properly administer Emgality.
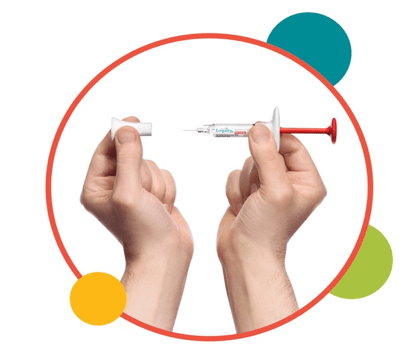
1. Uncap the syringe
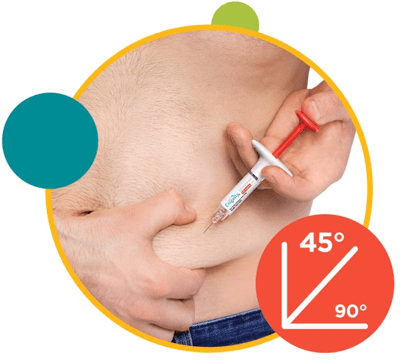
2. Insert the needle at a 45° angle into a gently pinched fold of skin
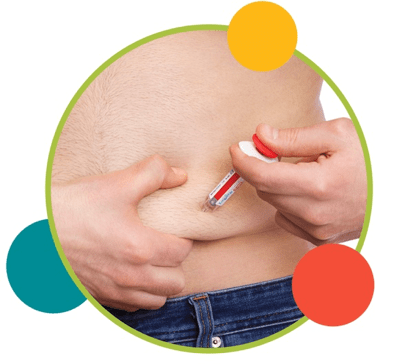
3. Inject slowly until the gray syringe plunger is pushed to the needle end of the syringe
SELECT IMPORTANT SAFETY INFORMATION
Adverse Reactions
The most common adverse reactions (incidence ≥2% and at least 2% greater than placebo) in Emgality clinical studies were injection site reactions.

Telephonic injection training and an injection how-to video are available to help guide your patients through self-administration.
Questions? Call 1-833-EMGALITY (1-833-364-2548).
Watch how to use the Emgality prefilled syringe
Instructions for Use - 100 mg syringe for episodic cluster headache
If you or your patients have any questions about Emgality, call 1-833-EMGALITY (1-833-364-2548) to talk to a healthcare provider.
SC=subcutaneous.
SELECT IMPORTANT SAFETY INFORMATION
Hypersensitivity Reactions
Hypersensitivity reactions, including dyspnea, urticaria, and rash, have occurred with Emgality in clinical studies and the postmarketing setting. Cases of anaphylaxis and angioedema have also been reported in the postmarketing setting. If a serious or severe hypersensitivity reaction occurs, discontinue administration of Emgality and initiate appropriate therapy. Hypersensitivity reactions can occur days after administration and may be prolonged.
References
- Emgality. Prefilled Syringe Prescribing Information. Lilly USA, LLC.
- Data on File. Lilly USA, LLC. DOF-GZ-US-0071.
- Emgality. Prefilled Syringe Instructions for Use. Lilly USA, LLC.