Discover what Emgality® can do for patients with chronic migraine1

For your patients with ≥15 headache days per month1
Emgality reduced mean monthly MHDs1
Emgality prevented significantly more mean monthly MHDs vs placebo1a:
- REGAIN: 4.8 with Emgality 120 mg (N=273) vs 2.7 with placebo (N=538) (baseline mean: 19.4 vs 19.6) over Months 1 to 3 (p<0.001)
Emgality demonstrated a reduction in MHDs in the first month and every following month1
Mean Change From Baseline in Monthly MHDs1,2a
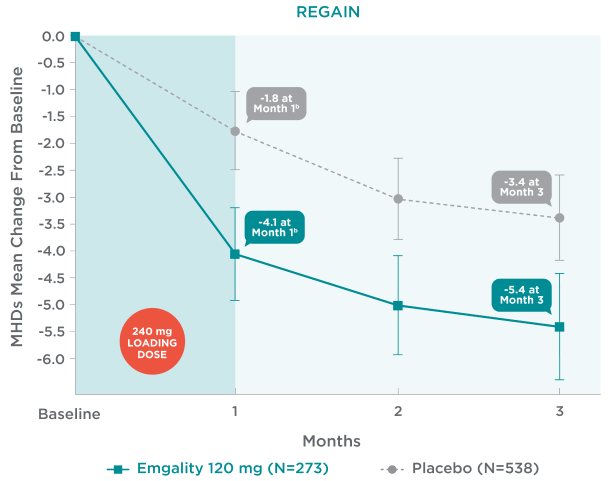
aLS means and 95% confidence intervals are presented.1-3
bEarliest post-baseline, prespecified assessment.
In REGAIN, mean change from baseline in monthly MHDs with Emgality 120 mg (N=273) plus 240 mg loading dose vs placebo (N=538), respectively1,2a:
- Month 1: -4.1b vs -1.8b
- Month 2: -5.0 vs -3.0
- Month 3: -5.4 vs -3.4
No formal hypothesis testing was conducted to evaluate treatment difference in mean monthly MHD reduction at each month. However, patients treated with Emgality 120 mg showed a nominally greater reduction in number of monthly MHDs at each month compared to placebo.1,2
See study design for REGAIN.
See MHD data for episodic migraine.
SELECT IMPORTANT SAFETY INFORMATION
Contraindications
Emgality is contraindicated in patients with serious hypersensitivity to galcanezumab-gnlm or to any of the excipients.
For your patients with ≥15 headache days per month1
With Emgality, a significantly greater mean percentage of patients achieved a ≥50% reduction in monthly MHDs from baseline vs placebo (p<0.001)1
≥50% Reduction of Monthly MHDs From Baseline vs Placebo Over Months 1 to 31
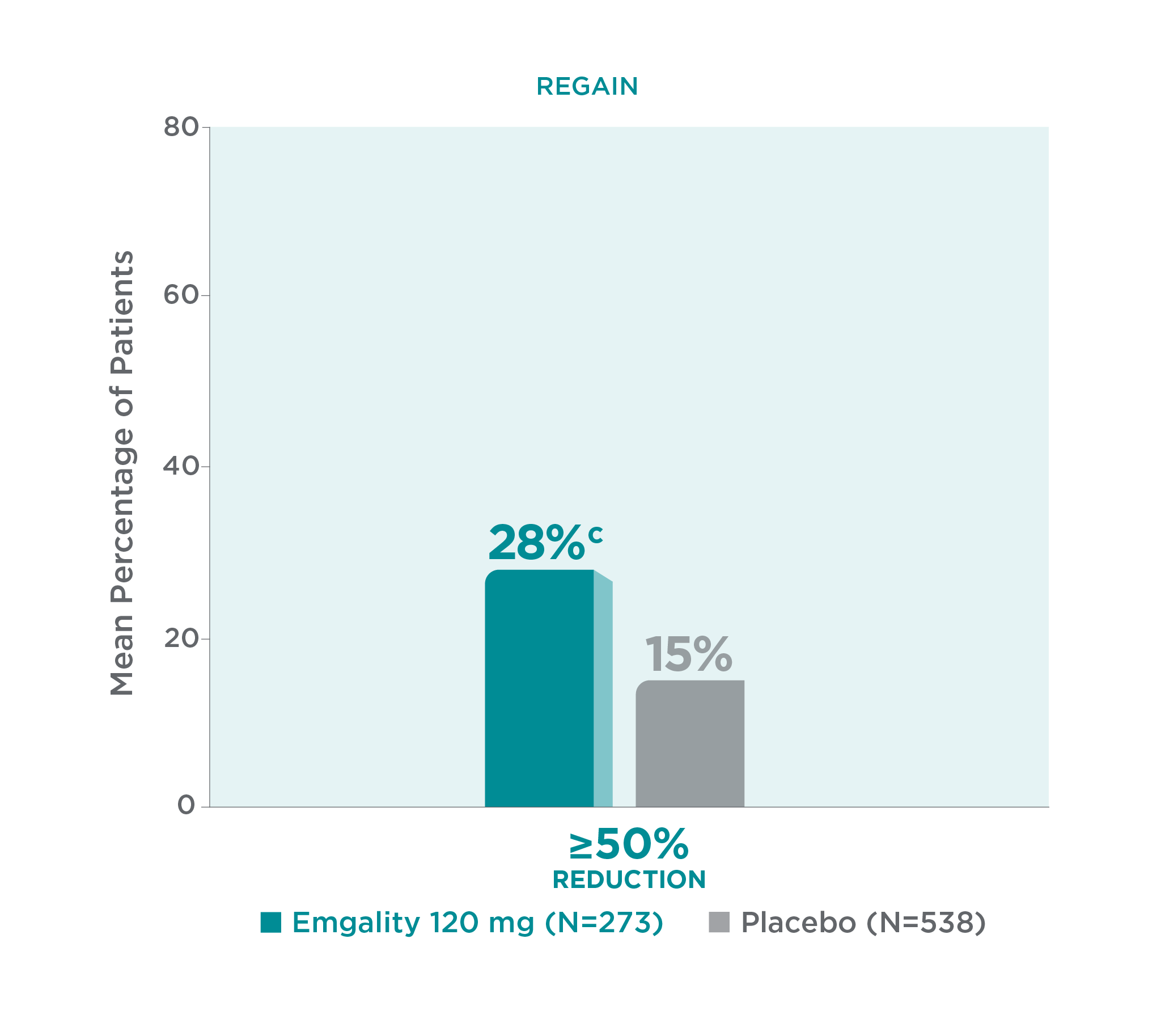
cp<0.001 vs placebo.1
LS=least-square; MHD=migraine headache day.
Twenty-eightc percent of patients with chronic migraine taking Emgality 120 mg (N=273) achieved a ≥50% reduction in monthly MHDs from baseline vs 15% with placebo (N=538) over Months 1 to 3 (p<0.001).1
In REGAIN, Emgality 120 mg was not significantly better than placebo for the mean percentage of patients with ≥75% or 100% reduction from baseline in the number of monthly MHDs over the 3-month treatment period.1
See study design for REGAIN.
See response rate for EVOLVE-1 and EVOLVE-2.
See data for the 12-month, open-label safety study.
SELECT IMPORTANT SAFETY INFORMATION
Hypersensitivity Reactions
Hypersensitivity reactions, including dyspnea, urticaria, and rash, have occurred with Emgality in clinical studies and the postmarketing setting. Cases of anaphylaxis and angioedema have also been reported in the postmarketing setting. If a serious or severe hypersensitivity reaction occurs, discontinue administration of Emgality and initiate appropriate therapy. Hypersensitivity reactions can occur days after administration and may be prolonged.
References
- Emgality. Prescribing Information. Lilly USA, LLC.
- Data on File. Lilly USA, LLC. DOF-GZ-US-0002.
- Data on File. Lilly USA, LLC. DOF-GZ-US-0120.