For the preventive treatment of migraine in adults1-3
Why add to your patients' pill burden?
For migraine prevention, once-monthly injectable Emgality doesn't require your patients to adhere to a daily or every-other-day oral gepant dosing schedule1-3
Doses per year1-3
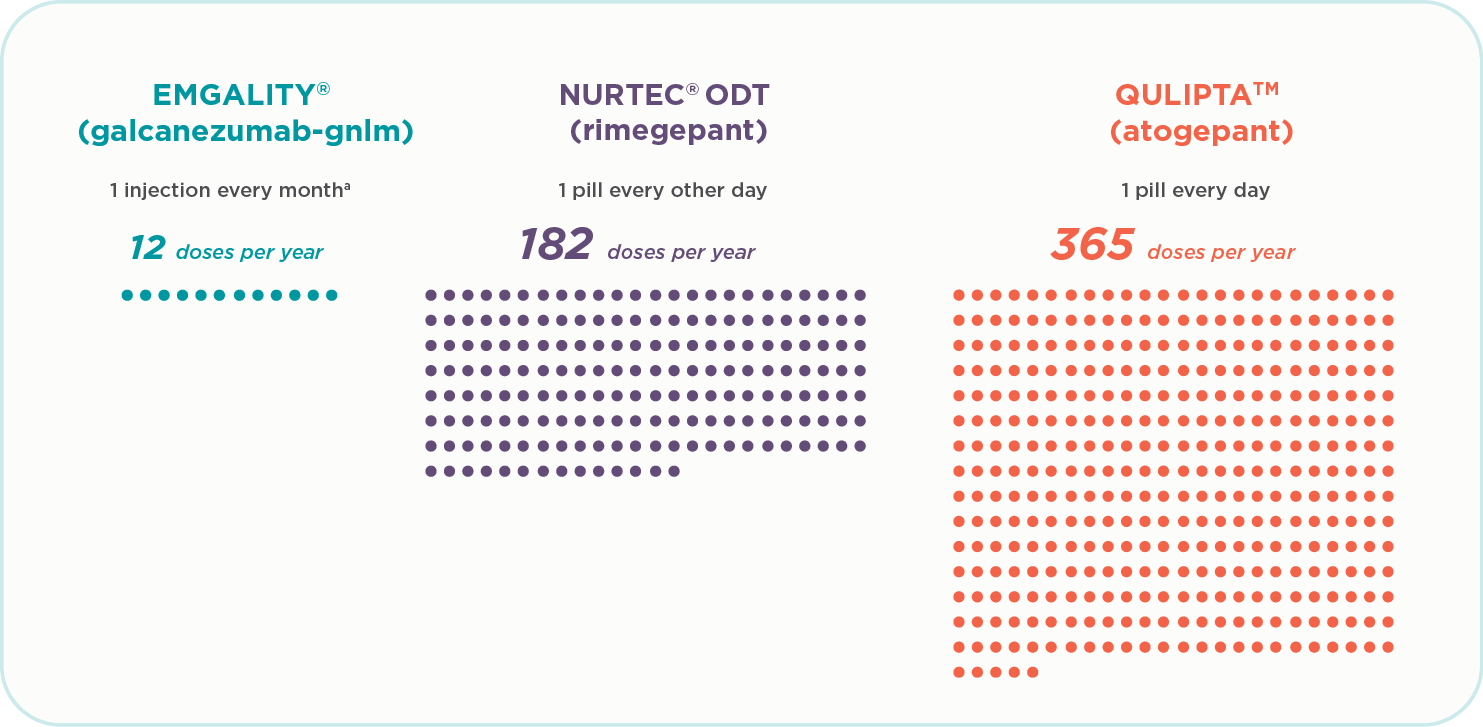
Doses during one year of therapy1-3:
- Emgality (galcanezumab-gnlm) is 12 doses a year (1 injection every month)a
- Nurtec ODT (rimegepant) is 182 doses a year (1 pill every other day)
- Qulipta (atogepant) is 365 doses a year (1 pill every day)
Patients on some oral preventive treatments may be taking up to 365 doses per year. Emgality is 12 doses per year.1-3a
Pill burden is defined by the total number of pills a patient takes on a daily or regular basis.4,5
aAfter an initial loading dose of 240 mg (2 x 120 mg injections).1
SELECT IMPORTANT SAFETY INFORMATION
Adverse Reactions
The most common adverse reactions (incidence ≥2% and at least 2% greater than placebo) in Emgality clinical studies were injection site reactions.
References
- Emgality. Prescribing Information. Lilly USA, LLC.
- Nurtec ODT. Prescribing Information. Pfizer Ireland Pharmaceuticals, Inc.
- Qulipta. Prescribing Information. AbbVie Inc.
- Chiu YW, Teitelbaum I, Misra M, et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089-1096.
- Pill burden definition. National Institutes of Public Health. Clinicalinfo.HIV.gov glossary. Accessed May 20, 2022. https://clinicalinfo.hiv.gov/en/glossary/pill-burden.