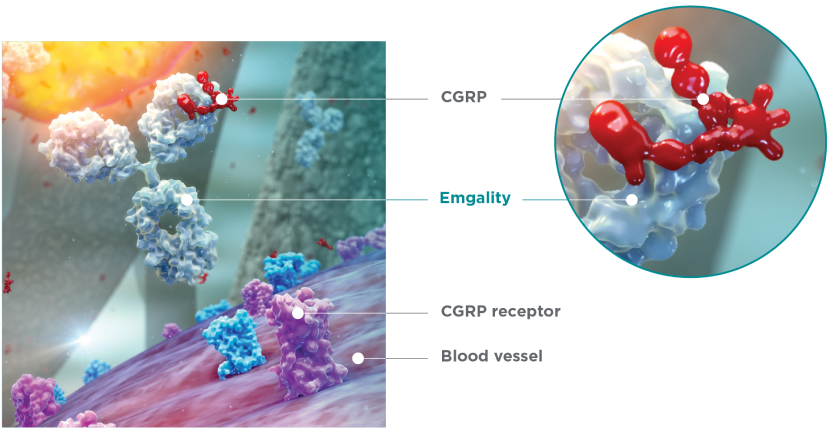
Emgality works differently than many other preventive medications1-3
Emgality is a humanized monoclonal antibody specficially developed to bind to CGRP1
There are no relevant data on the pharmacodynamic effects of galcanezumab-gnlm.1
SELECT IMPORTANT SAFETY INFORMATION
Contraindications
Emgality is contraindicated in patients with serious hypersensitivity to galcanezumab-gnlm or to any of the excipients.
CGRP is thought to play a key role in migraine4
Elevated levels of CGRP can lead to a cascade of vasodilation and pain associated with migraine attacks, as observed in preclinical studies of CGRP.4
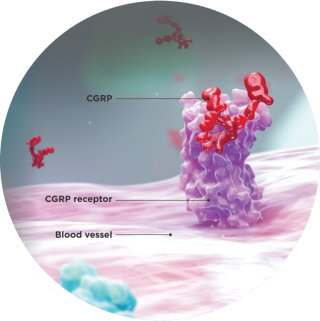
- CGRP, released from the trigeminal ganglia, is elevated during migraine attacks4
- Perivascular release of CGRP has been shown to induce vasodilation and plasma protein leakage from tissues4
- An infusion of CGRP can induce migraine attacks in susceptible individuals, and CGRP is involved in the transmission of pain5,6
CGRP=calcitonin gene-related peptide.
References
- Emgality. Prescribing information. Lilly USA, LLC.
- Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
- Garza I, Swanson JW. Prophylaxis of migraine. Neuropsychiatr Dis Treat. 2006;2(3):281-291.
- Durham PL. CGRP-receptor antagonists—A fresh approach to migraine therapy? N Engl J Med. 2004;350(11):1073-1075.
- Lassen L, Haderslev P, Jacobsen V, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54-61.
- Oku R, Satoh M, Fujii N, et al. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Research. 1987;403(2):350-354.