How to administer Emgality1
Preparation
- Check the pen to be sure it is not expired, damaged, cloudy, discolored, or has particles in it
- Choose an area for injection (abdomen or thigh if self-injecting or buttocks or back of upper arm if another person is injecting), being sure to choose a different site (even within an area) each month
- Clean the site with an alcohol wipe and let it dry before injecting
A simple approach: 3 key administration steps
Please review the full Instructions for Use with your patients to ensure they understand how to properly administer Emgality.
1. Uncap the pen
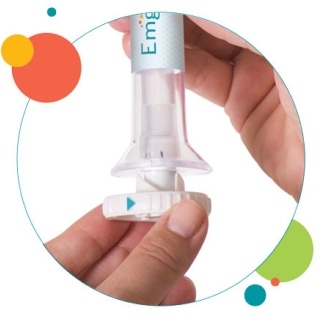
2. Place and unlock

3. Press and hold

SELECT IMPORTANT SAFETY INFORMATION
Contraindications
Emgality is contraindicated in patients with serious hypersensitivity to galcanezumab-gnlm or to any of the excipients.
Watch how to use the Emgality Pen
00:06-00:15
[Acoustic music plays in background, white Emgality logo appears on green background]
Caption: INSTRUCTIONS FOR INJECTING ONE DOSE OF EMGALITY (120 MG)
Caption: Do not use Emgality if you are allergic to galcanezumab-gnlm or any of the ingredients in Emgality.
Caption: Please see Purpose and Safety Summary at the end of this video.
Narrator: The following instructions are for the Emgality pen. For information about the prefilled syringe, and for additional information about the pen...
00:15-00:24
Caption: INSTRUCTIONS FOR INJECTING ONE DOSE OF EMGALITY (120 MG) FOR THE PREVENTIVE TREATMENT OF MIGRAINE
Caption: Do not use Emgality if you are allergic to galcanezumab-gnlm or any of the ingredients in Emgality.
Caption: Do not inject until after you have been shown how to inject Emgality by your healthcare provider.
Caption: If you have vision or hearing problems, do not use the Emgality Pen without help from a caregiver.
Narrator: ...please see the instructions for use that come in the Emgality box. If you have vision or hearing problems, do not use the Emgality pen without help from a caregiver.
00:25-00:37
[We see Jessica arrive home, take the pen out of the refrigerator, and place it on the table.]
Narrator: This video, in addition to the instructions for use, will help you get started. Take the pen from the refrigerator and leave it at room temperature for at least 30 minutes.
00:38-00:39
[Upon mention of "microwave," the foreground subtly blurs and the camera focuses on the microwave. An "X" moves on-screen. It stops on the microwave, indicating that the pen cannot be microwaved.]
Narrator: Do not microwave the pen, run hot water over it, or leave it in direct sunlight. Also, do not shake it or freeze it.
00:45-00:48
[Camera shows Jessica finishing washing her hands.]
Wash your hands before preparing the injection site.
00:49-1:03
[CUT TO: Jessica looking at the pen in her kitchen.]
Narrator: If this is your first dose of Emgality, you will be injecting a loading dose of 240 mg or two pens. Your first dose may be administered at your doctor's office. After that, you can inject one dose or pen on your own each month.
01:03-01:19
[Camera zooms in on the pen being inspected.]
Narrator: Inspect the pen to ensure it does not look damaged. The medicine inside should be colorless to slightly yellow to slightly brown. Do not use if it is frozen, cloudy, or discolored, or contains any particles. Be sure to check the expiration date before administering.
01:19-01:43
[She then cleans the injection site]
Caption: Do not inject where the skin is tender, bruised, red, or hard.
Narrator: Choose an injection site such as the abdomen that is at least 2 inches away from the belly button. You may also inject in the front of your thighs, at least 2 inches above the knee and 2 inches below the groin. Another person may administer Emgality in the back of your upper arm or buttocks. Make sure that you clean the injection side with an alcohol wipe and let it dry before administering Emgality. Do not inject in the exact same spot twice.
01:44-01:56
[We see Jessica uncapping the pen and placing it over the chosen spot.]
Caption: Be sure not to touch the needle.
Narrator: Now that your pen is ready, here are the three basic steps to administer your injection. First, make sure the pen is in the locked position. Then uncap the pen by twisting off the base cap and throw it in the trash.
01:58-02:22
[She holds the pen against her skin as a countdown clock graphic appears in the bottom right, starting at 10 seconds. She presses the button for the first click and the clock starts counting down. When the clock hits "0," we hear the second click.]
Narrator: Second, place and hold the clear base flat and firmly against your skin. Turn the lock ring to the unlock position. The third step is to press and hold the teal injection button. You will hear a loud click. Keep holding the clear base firmly against your skin. About 10 seconds after the first click, you will hear a second click and the gray plunger will be visible. This means your injection is complete.
02:22-02:27
[We see Jessica throwing the pen away in a sharps container.]
Caption: Do not throw away the Emgality Pen in your household trash.
Narrator: After the injection, put the used pen in a sharps container.
02:27-02:35
[We see Jessica continuing her day after disposing of the pen in a sharps container.]
Narrator: Now that you've viewed the basic steps, remember to read and follow the instructions for use when you use Emgality each month.
02:37-02:54
Caption: Consumer Purpose and Safety Summary scroll.
Narrator: Purpose and safety summary. Important facts about Emgality (em-GAL-it-ē) injection, also known as galcanezumab-gnlm. Emgality is a prescription medicine used for the preventive treatment of migraine in adults. The medicine comes in a prefilled pen or syringe and is taken once a month.
02:54-3:23
Warnings: Do not use Emgality if you are allergic to galcanezumab-gnlm or any of the ingredients in Emgality. Emgality may cause allergic reactions such as itching, rash, hives, and trouble breathing. Allergic reactions can happen days after using Emgality. Call your healthcare provider or get emergency medical help right away if you have any of the following symptoms which may be part of an allergic reaction: swelling of your face, mouth, tongue or throat, or trouble breathing.
03:23-03:43
Common side effects: The most common side effects of Emgality are injection-site reactions. These are not all the possible side effects of Emgality. Tell your doctor if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
03:43-03:58
Before using: Before you use Emgality, discuss these questions with your doctor. Do you have other medical conditions? Do you take other medicines? Tell your doctor about all the prescription and over the counter drugs, vitamins and herbal supplements you take.
03:58-04:27
Tell your doctor if: You are pregnant or breastfeeding. Pregnancy Registry. There is a pregnancy registry for women who take Emgality. The purpose of this registry is to collect information about the health of you and your baby. You may enroll yourself by calling 1-833-464-4724 or by visiting www.migrainepregnancyregistry.com or you may talk to your healthcare provider about how you can take part in this registry.
04:28-04:32
You are under age 18: It is not known if Emgality is safe and effective in children.
04:32-04:51
How to take: Read the instructions for use that come with Emgality. There you will find information about how to store, prepare, and inject Emgality. Use Emgality exactly as your doctor says. If you miss a dose, inject it as soon as possible, then continue to take it once a month from the date of your last dose.
04:52-05:22
Learn more: For more information, go to www.emgality.com or call 1-833-emgality (1-833-364-2548). This summary provides basic information about Emgality, but it does not include all information known about this medicine. Read the information that comes with your prescription every time you have a prescription filled. Talk to your doctor or pharmacist about this information. Your doctor is the best person to help you decide if Emgality is right for you.
05:22-05:23
[The Emgality and Lilly logos appear on screen.]
Caption: Emgality is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries, or its affiliates. ©Lilly USA, LCC 2022. All rights reserved. GZ CON MIG BS 11MAR2021
Caption: Please see Instructions for Use that come with the device.
Telephone injection training is also available to help guide your patients through self-administration. Call 1-833-EMGALITY (1-833-364-2548).
SELECT IMPORTANT SAFETY INFORMATION
Adverse Reactions
The most common adverse reactions (incidence ≥2% and at least 2% greater than placebo) in Emgality clinical studies were injection site reactions.
Reference
- Emgality. Autoinjector Instructions for Use. Lilly USA, LLC.